By Benjamin Mateus
26 May 2020
Last week, Moderna’s stocks skyrocketed 30 percent after the media touted the company’s announcement that eight participants in its coronavirus vaccine trial had elicited neutralizing antibodies after receiving the mRNA vaccine as a cure for the pandemic. The Dow shot up over 900 points in the resulting stock market frenzy, and Moderna’s stock rose to a peak of $87 per share before it settled at above $80 on closing. The company’s value swelled to $29 billion despite not having had any products in the markets to date.
Over the weekend, CNN Business reported that Moderna’s chief financial officer, Lorence Kim, “exercised 241,000 options for $3 million on Monday. He then immediately sold them for $19.8 million, creating a profit of $16.8 million.” In a similar maneuver, Moderna’s chief medical officer, Tal Zaks, spent $1.5 million on options, then immediately sold the shares back for $9.77 million, netting a profit of $8.2 million.
 Moderna's Norwood plant
Moderna's Norwood plant
These payoffs are dwarfed, however, by the killing made by CEO Stéphane Bancel, who had already become a billionaire in March, while the global economy was facing an implosion, based on the valuation of his nine percent stake in the publicly-traded company.
Notwithstanding the parasitic and avaricious behavior of these senior executives, these maneuvers do not appear to raise any legal red flags. In actuality, these actions are considered typical. Charles Whitehead, professor at Cornell Law School, said, “On its face, there is nothing wrong with these trades. It’s what a 10b5-1 plan is intended for, assuming the requirements are met.” The plan, created under the Securities and Exchange Act of 1934, allows major holders to sell a predetermined number of shares at a predetermined time. Corporate executives use this plan to avoid accusations of insider trading.
The CNN report confirms that the release of the “premature data” was designed and intended to raise the share price of Moderna’s stock so that executives could cash in during the period of euphoria. Since Moderna was first established in 2010, the business model of this biotech firm has been based on speculation and promises that it will produce a vaccine for a number of viruses—Zika, respiratory syncytial virus, influenza, to name a few. Moderna has yet to deliver on any of its grandiose claims.
The claims in relation to COVID-19, while couched in technical and business jargon, were equally sweeping.
“These interim Phase 1 data, while early, demonstrate that vaccination with mRNA-1273 elicits an immune response of the magnitude caused by natural infection starting with a dose as low as 25 [micrograms (mcg)],” said Tal Zaks, multi-millionaire Chief Medical Officer at Moderna. “When combined with the success in preventing viral replication in the lungs of a pre-clinical challenge model at a dose that elicited similar levels of neutralizing antibodies, these data substantiate our belief that mRNA-1273 has the potential to prevent COVID-19 disease and advance our ability to select a dose for pivotal trials.”
“With today’s positive interim Phase 1 data and the positive data in the mouse challenge model, the Moderna team continues to focus on moving as fast as safely possible to start our pivotal Phase 3 study in July and, if successful, file a BLA,” said CEO Bancel, referring to a Biologics License Application. “We are investing to scale up manufacturing so we can maximize the number of doses we can produce to help protect as many people as we can from SARS-CoV-2.”
The real goal, however, is not to “help protect as many people as we can,” but rather to make as much money as possible, as fast as possible, regardless of the consequences for test subjects, future patients, or the world as a whole.
Bancel’s claim of a summer start contradicts the fall 2020 start noted in the Biomedical Advanced Research and Development Authority (BARDA) contract with Moderna. BARDA is a division of the US Department of Health and Humans Services Office of the Assistant Secretary for Preparedness and Response.
And not more than 36 hours had passed before the initial euphoria had subsided, and more sober analysts began to pick through the sparse press release data provided to the media. Moderna’s stocks began a downward slide to close at $67 a share.
Moderna’s vaccine trial claim
On Monday, May 18, Moderna announced, “After two doses, all participants evaluated to date across the 25-mcg and 100-mcg dose cohorts seroconverted with binding antibody levels at or above levels seen in convalescent sera. mRNA-1273 elicited neutralizing antibody titer levels in all eight initial participants across the 25-mcg and 100 mcg dose cohorts, reaching or exceeding neutralizing antibody titers generally seen in convalescent sera.” They then prefaced these remarks with “mRNA provided full protection against viral replication in the lungs in a mouse challenge model” to cover for the lack of any real clinical relevance to their press release.
The next day, STAT News wrote, “the NIAID [National Institute of Allergy and Infectious Diseases] did not put out a press release Monday and declined to provide comment on Moderna’s announcement … the report of neutralizing antibodies in subjects who were vaccinated comes from blood drawn two weeks after they received their second dose of vaccine. Two weeks, ‘that’s very early. We don’t know if those antibodies are durable,’ said Anna Durbin, a vaccine researcher at Johns Hopkins University.” STAT also questioned the statement that levels of antibodies seen were at or above the levels of the sera of recovered patients. They wrote, “among 175 recovered COVID-19 patients studied, 10 had no detectable neutralizing antibodies.”
Since April, Moderna has made several amendments to its vaccine trials. They have added older age groups to their phase 1 trial. Another change included 50-mcg dosing. The phase 2 trial will also include a 50-mcg as well as 100-mcg dose levels to select a dose for the phase 3 trial. Animal experiments are now being conducted in parallel when they had initially been considered unnecessary.
The mRNA-1273 is a novel lipid nanoparticle (LNP)-encapsulated vaccine that encodes for a perfusion stabilized form of the viral Spike protein. The target was selected by investigators from the Vaccine Research Center (VRC) at the NIAID. On May 6, the FDA reviewed and approved Moderna’s Investigational New Drug application (submitted on April 27), allowing it to proceed to a phase 2 study. On May 12, the vaccine was given a Fast Track designation.
FDA clears Moderna Phase II trial for COVID-19
The company’s Phase 2 trial is scheduled to start in June. It is ostensibly designed to assess the safety, reactogenicity, and immunogenicity of two doses of mRNA-1273 given four weeks apart. The three trial groups will include a set of patients taking a placebo, a set taking 50 mcg of the vaccine, and a group taking 100-mcg of the vaccine, pending finalization of the phase 1 trial. The intent of this phase 2 is to enroll 600 participants, 300 in the 18 to 55 age category, and 300 in the over 55 age category .
On April 16, BARDA provided Moderna with $483 million in funds to accelerate the development of the company’s mRNA Vaccine in support of late-stage clinical development programs to see it quickly to FDA licensure.
“We are thankful for BARDA’s support to fund the accelerated development of mRNA-1273, our vaccine candidate against SARS-CoV-2,” said Moderna CEO Bancel. “Time is of the essence to provide a vaccine against this pandemic virus. By investing now in our manufacturing process scale-up to enable large scale production for pandemic response, we believe that we would be able to supply millions of doses per month in 2020 and with further investments, tens of millions per month in 2021, if the vaccine candidate is successful in the clinic.”
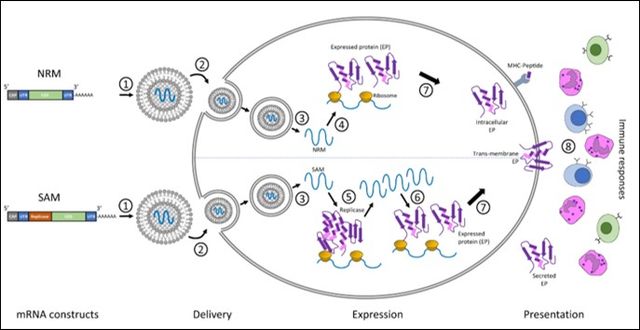 mRNA Vaccine Diagram and Mechanism: 1) the genetic sequence is formulated and encapsulated in a lipid nanoparticle. 2&3) The vaccine particle is taken up by the cell and the mRNA strand is released into the cytosol. 4) The ribosomes translate the mRNA strand into the protein of interest. 5) Replicase enzymes are also produced to assist in self-amplifying the mRNA. 6) The self-amplified mRNA constructs then produce more protein of interest. 7) The proteins undergo post-translation modification and are secreted as membrane proteins. 8) The innate and adaptive immune responses detect the protein of interest.
mRNA Vaccine Diagram and Mechanism: 1) the genetic sequence is formulated and encapsulated in a lipid nanoparticle. 2&3) The vaccine particle is taken up by the cell and the mRNA strand is released into the cytosol. 4) The ribosomes translate the mRNA strand into the protein of interest. 5) Replicase enzymes are also produced to assist in self-amplifying the mRNA. 6) The self-amplified mRNA constructs then produce more protein of interest. 7) The proteins undergo post-translation modification and are secreted as membrane proteins. 8) The innate and adaptive immune responses detect the protein of interest.
At the beginning of May, Moderna signed a 10-year manufacturing agreement with the Lonza Group to initially build vaccine production suites at their facilities in the US and Switzerland, expanding later to Lonza’s worldwide facilities. The Lonza Group is a Swiss multinational, chemicals, and biotechnology company, headquartered in Basel. According to Genetic Engineering& Biotechnology News, the scale of the production is the produce 1 billion doses of mRNA-1273 each year at an expected dose of 50-mcg.
Bancel said, “This long-term strategic collaboration agreement will enable Moderna to accelerate, by 10-times, our manufacturing capacity for mRNA-1273 and additional products in Moderna’s large clinical portfolio. Lonza’s global presence and expertise are critical as we scale at unprecedented speed.”
Before even the initial data was available on the participants of the phase 1 aspect of their trial, Moderna and Lonza had announced that technology transfer would begin in June with the first batch of vaccines produced at Lonza’s US site in July before their phase 2 trial could be completed.
A “Shark Tank” approach to vaccine selection
According to a CNN exposé, three weeks before the US had proceeded into lockdowns, on March 2, President Trump was auditioning vaccine developers who were asked to pitch their product as a viable “cure” to battling the pandemic. John Shiver of Sanofi Pasteur vaccines proposed a vaccine that would take several years to bring for public use. Next, Lenny Schleifer, CEO of Regeneron, offered his semi-confident pitch, “clinical trials in the summer and producing 200,000 doses per month starting in August.” Trump then jumped in and said, “So, the process would be faster than John’s?” Trump’s main concern was with speed (with an eye to both the stock market and the presidential election) and not efficacy or scientific rationale.
In January 2020, once it was discovered that the infection in Wuhan was caused by a novel coronavirus, Bancel quickly emailed Dr. Barney Graham, deputy director of the Vaccine Research Center at the National Institutes of Health, asking him to send the genetic sequence for the virus. On February 24, in 42 days, Moderna had produced a batch of vaccine and sent it to the NIH. Discussions at the time projected a 12-to-18-month window to determine if this new vaccine was safe and effective. Moderna added that they could have a vaccine ready for emergency use by the fall of 2020.
So, when Bancel’s turn came up to sell his vaccine, with Dr. Fauci present at the briefing, he said, “I’m very proud to be working with the US government and to have already sent, in only 42 days from the sequence of the virus, our vaccine to Dr. Fauci’s team at the NIH.” He omitted the few months to phase 2 trials or a 12-to-18-month timeline that had been discussed the week prior. It was a sufficient omission to hook the president. Fauci interjected, “You won’t have a vaccine. You’ll have a vaccine to go into testing.” But the deal was already made.
The federally funded trial was rapidly rubber-stamped through regulatory agencies, and human trials commenced on March 16. Unsettlingly, the regulatory agencies skipped the traditional but necessary animal trials, a critical path in testing the efficacy of the virus before injecting it into human subjects. Akiko Iwasaki, a microbiologist at Yale University, told STAT News, “This is very unusual. It reflects the urgency to develop vaccines to counter COVID-19 pandemic.”
Mark Feinberg, president and CEO of the International AIDS Vaccine Initiative, said, “When you hear predictions about it taking at best a year or a year and a half to have a vaccine available … there’s no way to come close to those timelines unless we take new approaches. I personally think that’s not only appropriate; I think that’s the only option we have.”
This is entirely false. Skipping animal trials and other corner-cutting to speed development of a vaccine that may be given to the entire human race is not being driven by health concerns. In that event, a crash program to develop vaccines would be combined with a continuing and long-term lockdown to save as many lives as possible.
Instead, the vaccine development—now at “warp speed” according to Trump—is being combined with a general reopening of the economy and relaxation of measures like social distancing, which mean that hundreds of thousands, if not millions, will die before any vaccine can possibly be developed, no matter how intensive the effort.
There are two concerns driving the vaccine development. On the part of Moderna and other biotech companies, it is sheer greed. Whichever company gets its vaccine to the market first will reap monopoly profits. On the part of the Trump administration and other capitalist governments around the world, there is an additional factor: gaining a geopolitical advantage over their rivals by being first to develop a vaccine, and perhaps even withholding the vaccine as part of efforts to assert American dominance.
These reactionary considerations are the real motive for efforts to bypass safety regulations that would potentially cause harm to the population. According to Jonathan Kimmelman, director of McGill University’s biomedical ethics unit, “Outbreaks and national emergencies often create pressure to suspend rights, standards and, or, normal rules of ethical conduct. Often our decision to do so seems unwise in retrospect.”
https://www.wsws.org/en/articles/2020/05/26/vacc-m26.html
Counter Information published this article following the Creative Commons rule. If you don't want your article to appear in this blog email me and I will remove it asap.




















No comments:
Post a Comment